Did you know surfactants and other inhibitory compounds commonly found in wastewater treatment plants are causing serious problems? Until recently, there wasn’t a viable solution to manage these compounds. Moleaer’s nanobubble technology has emerged as a clean-air chemistry to selectively break down and remove these compounds to help wastewater treatment plants solve the issues they cause.
WHAT ARE SURFACTANTS?
You may not know what surfactants are, but you certainly are familiar with what they do. These chemical compounds are ubiquitous in modern life. The word surfactant comes from “SURFace ACTive AgeNT,” which describes their chemical function: they lower the surface tension between two substances, such as a liquid and solid or two unmixable liquids.
The structure of surfactants typically consists of a hydrophilic (water-loving) head and a hydrophobic (water-repelling) tail. This unique structure enables surfactants to interact simultaneously with both water and non-water substances, facilitating the formation of stable mixtures or emulsions.
Surfactants are used widely in various industries, including personal care products, detergents, cosmetics, pharmaceuticals, paints and coatings, agriculture, and oil recovery processes. Common examples include sodium lauryl sulfate (SLS) and benzalkonium chlorides (BACs), used in industrial cleaning. They contribute to the functionality, stability, and performance of these products and processes by modifying interfacial properties and improving interactions between different substances.
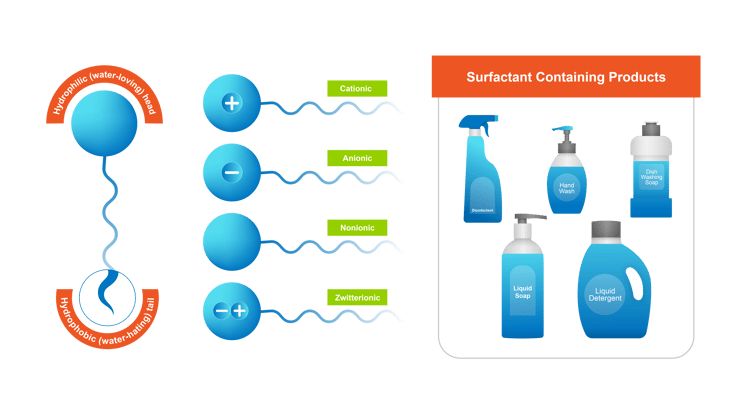
Although beneficial for many applications, the use of surfactants has negative consequences in wastewater treatment and the environment. According to a recent paper published in the journal Advances in Colloid and Interface Science, “[Surfactants] destroy aquatic microbial populations, damage fish and other aquatic life, and reduce photochemical conversion efficiency of plants as well as adversely affecting wastewater treatment processes.”
HOW DO SURFACTANTS INHIBIT WASTEWATER TREATMENT PROCESSES?
In addition to their harmful accumulation in the environment, surfactants impede wastewater treatment processes in both industrial and municipal waste streams.
When mixed in water with fats, oils, and grease (FOG), surfactants increase the complexity and cost of wastewater treatment because they interfere with nearly all water and wastewater treatment processes (physical, biological, and chemical). FOG and surfactants disrupt water treatment because they emulsify wastewater, impede oxygen transfer, interfere with separation, inhibit biological processes, and consume disinfection chemicals. They also disrupt advanced water treatment (AWT) by causing pass through of FOG, other colloidal materials, and total organic carbon to Advanced Water Treatment Facilities (AWTFs). In AWT, FOG and surfactants cause issues like accelerated fouling of micro/ultra-filtration and reverse osmosis membranes, requiring more energy for backwashing and more chemicals for clean-in-place procedures. In some cases, the presence of these contaminants restricts the extent of AWT and water recycling that a municipality can cost-effectively achieve.
Surfactants have an affinity to adsorb to the solids surface - a key characteristic for a cleaning agent. In wastewater treatment, they sorb to the sludge that is then sent to anaerobic digester where they cannot be treated. In addition to creating inhibition in the anaerobic digestion process, they create foaming issues and reduced biogas quality.
Effects of High Levels of Surfactants in Wastewater Treatment Plants
Most aerobic biological processes in wastewater treatment can manage low levels of surfactants and can be effective in removing them. However, at high concentrations, they can be toxic to the biology in aerobic treatment. Additionally, they:
- Inhibit activated sludge processes
- Reduce oxygen transfer efficiency
- Lower oxygen transfer in the biomass
- Reduce biomass kinetics
- Interfere with solids separation and dewaterability of the sludge
Process upsets, poor treatment efficiency and reduced treatment capacity are often caused by toxic and inhibitory substances like surfactants. These issues have become more prevalent in recent years due to increasing surfactant concentrations in wastewater. Several recent trends have resulted in elevated surfactant concentrations in wastewater making surfactant removal more important than ever.
- The pandemic and trends in surfactant-containing products. During the height of the COVID-19 pandemic, people were urged to wash and disinfect their homes, workspaces, schools, hospitals, stores, and themselves more frequently and more thoroughly, leading to higher concentrations of surfactants in wastewater discharged to the collection system. The move toward liquid soaps and concentrated liquid detergents and away from bar soaps and powdered detergents, a trend that preceded COVID, has also exacerbated the problem because liquid cleaners contain more surfactants than solid cleaners.
- Water conservation. Paradoxically, the success of water conservation efforts, plus the introduction of high-efficiency appliances that use less water, has also led to greater challenges for wastewater treatment facilities. While consumers are using less water to accomplish their household and cleaning tasks, which is beneficial from a climate perspective, they continue to use the same amount of soap and cleaning products. So, less water is being added to the collection system resulting in higher concentrations of surfactants in wastewater.
- Increasing severity and length of droughts are also contributing to the problem. As water becomes scarcer, people are forced to conserve more water, resulting in less water in the collection system and higher concentration of surfactants in wastewater.
Nanobubble Technology: Combatting the Impact of Surfactant Concentration in Wastewater Treatment Plants
This situation has put immense strain on the performance and operations of wastewater resource recovery facilities (WRRFs) and wastewater treatment plants as they’re seeing increasingly more frequency in process upsets. Many of the symptoms of surfactants are often misdiagnosed as insufficient treatment capacity requiring costly infrastructure improvements to address. Alternately, some plant operators solve the problem with temporary fixes such as applying chemicals like quat blockers without long-term mitigation.
Moleaer’s nanobubble technology offers wastewater treatment plants a non-toxic pretreatment method that removes surfactants from wastewater and improves the efficiency of downstream treatment processes including primary treatment, secondary treatment, and disinfection.
Nanobubbles have unique properties that allow for use across multiple applications and deliver significant value to our customers and the environment. In wastewater treatment, nanobubbles work like clean chemistry – changing the fundamental nature of the inhibitory compounds and delivering significant results. Some of the specific nanobubble properties beneficial to wastewater treatment include:
- Size: The nanobubble has a diameter of <200 nanometers. Compared to a typical 1-mm fine bubble, these bubbles provide 10,000 times more surface area for adsorbing inhibitory compounds.
- Charged: Nanobubbles have a strong negative surface charge preventing them from coalescing and they spread throughout the water body.
- Stable: Nanobubbles are neutrally buoyant – they remain suspended in water giving the time for the reaction to happen.
- Hydrophobic: Nanobubbles are hydrophobic – and as such will attract the hydrophobic tails of the amphiphilic compounds.
- Internal Pressure: The nanobubble has a diameter of 100 nanometers. As determined by the Young Laplace equation, these bubbles have a high internal pressure of 400 psi (27.5 bar). Therefore, these bubbles release tremendous amounts of pressure and heat energy when destabilized.
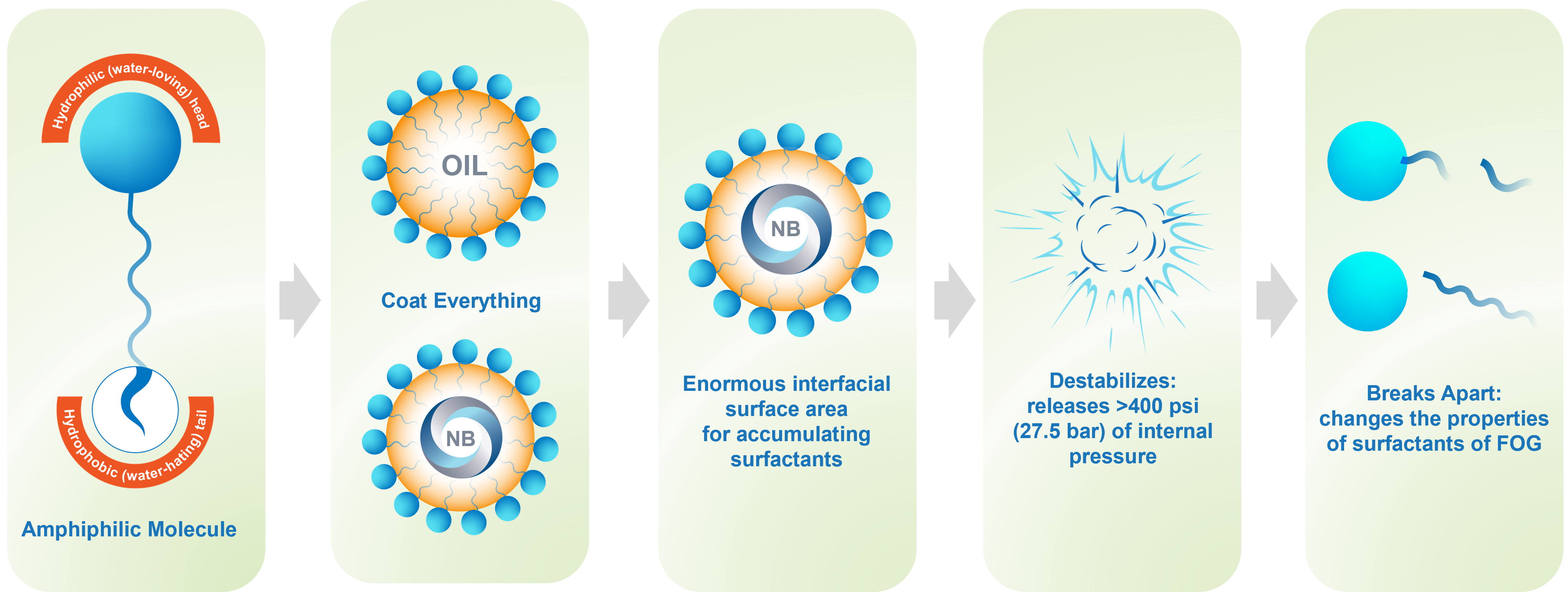
When introduced early in the wastewater process, the unique properties described above help the bubbles attract and sequester the inhibitory compounds. When destabilized, the bubble releases the energy trapped in it which breaks the surfactant molecules and makes them easy to treat. The inhibitory compound’s properties are fundamentally changed thus making them easier to treat. By changing their ability to coat other organic compounds, the remainder of the wastewater becomes easier to treat.
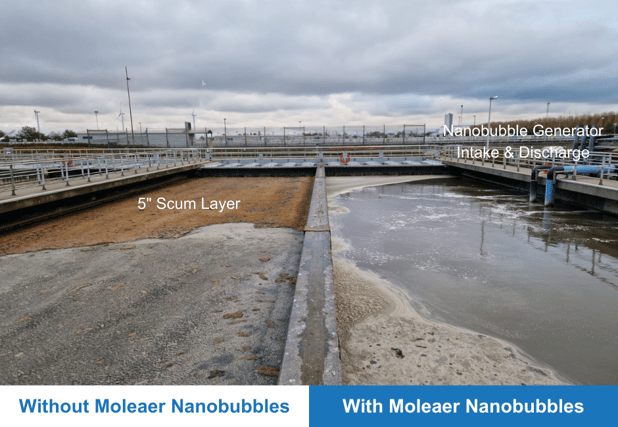
A Future with Nanobubble Pre-Treatment at Wastewater Treatment Plants
In conclusion, the increased concentrations of surfactants influent wastewater streams have led to detrimental effects on wastewater treatment processes, causing disruptions, inefficiencies, and environmental harm. The increasing concentrations of surfactants in wastewater, exacerbated by recent trends such as the Covid-19 pandemic, water conservation efforts, and prolonged droughts, have created challenges for wastewater treatment plants. However, Moleaer's nanobubble technology emerges as a solution to combat surfactants, offering a non-toxic pretreatment method to effectively remove surfactants and enhance the efficiency of downstream treatment processes. With unique properties such as size, charge, stability, hydrophobicity, and internal pressure, nanobubbles play a crucial role in altering the nature of inhibitory compounds, providing a sustainable and innovative approach to address the surfactant-related issues plaguing wastewater treatment plants. By embracing nanobubble technology, we can pave the way for cleaner, more efficient, and environmentally friendly wastewater treatment practices.


